Half the Genome:
Infinite Potential
The cells in our bodies are diploid, carrying 46 chromosomes, two sets of 23 chromosomes. One set is inherited from the mother, and the other, from the father. The only cells that contain a single set of chromosomes (Haploid cells) are post-meiotic germ cells, namely egg or sperm. While diploid cells can undergo mitosis and give rise to additional diploid cells, haploid germ cells are terminally differentiated cells incapable of producing progeny by cell division.
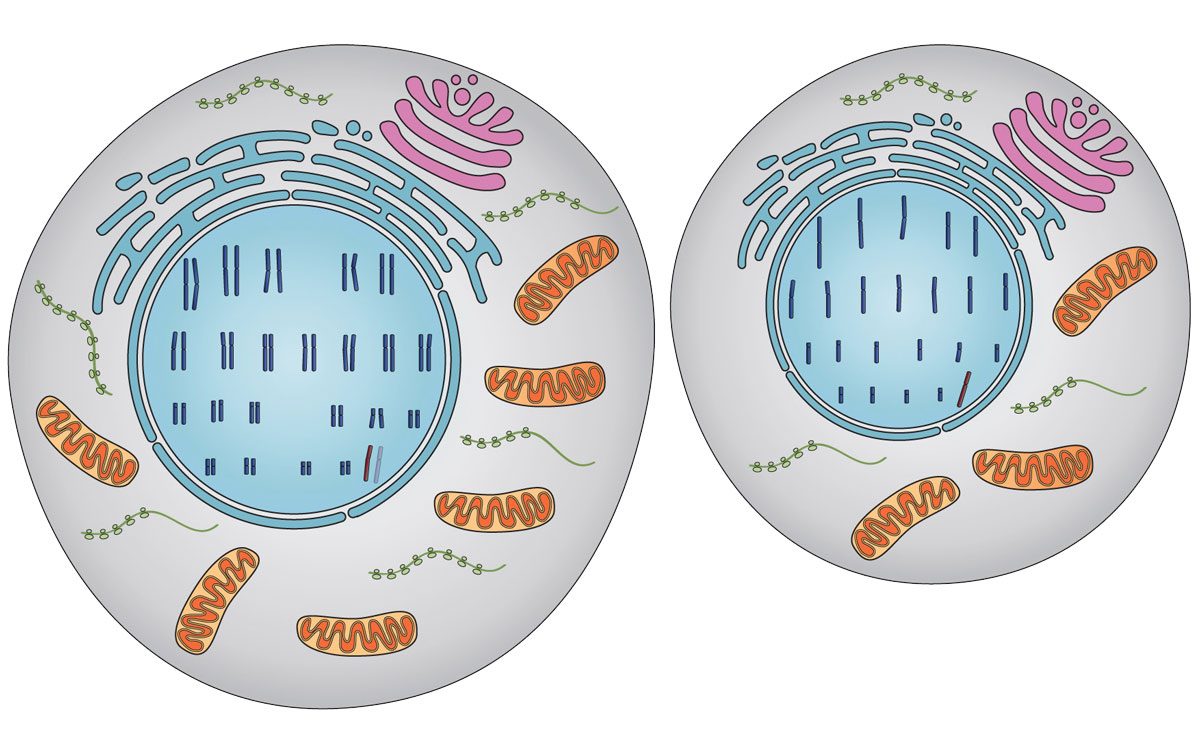
Diploid and haploid cells
Our technology has changed the dogma and enables what was formerly impossible: haploid human eggs can now be artificially induced to divide as early haploid embryos, allowing for the derivation of self-renewing haploid human embryonic stem cells (ESCs). Prof. Benvenisty and his team have isolated Haploid human ESCs, which readily differentiate into cells of all three embryonic germ layers while remaining haploid. With the combined advantages of haploid genetics and pluripotency, Haploid human ESCs now pave the way for numerous new biomedical applications in developmental biology, reproductive medicine, and regenerative medicine.
One of the most prominent biomedical tools is genetic screening. Screening for loss-of-function (LOF) phenotypes is rather limited in diploid cells, because if a mutation introduced into one allele, the second intact allele may serve as a backup. Therefore, recessive mutations are likely to be underrepresented among the screened phenotypes in diploid cells. For this reason, many global LOF screens were performed in haploid yeast, and only now when the derivation of haploid human ESCs, are available, it enables similar screens in human.
Genome-wide mutations can be introduced into haploid human ESCs by different methodologies. Libraries of mutant cells can then be subjected to different treatments followed by analysis of surviving clones, aiming to identify genes whose loss affords a growth advantage (positive selection) or disadvantage (negative selection). A similar approach is also applicable for haploid ESCs already carrying specific mutations, which can be isolated either from diseased eggs or generated by genome editing. In such cells, genome-wide mutagenesis can be performed to identify synthetic lethality interactions between the pre-existing mutation and a novel mutation within the library, based on mutual exclusivity. Alternatively, using diseased haploid human ESCs can reveal genes whose loss leads to reversion of the disease phenotype.
The Clear 23.
One of the most prominent biomedical tools is genetic screening. Screening for loss-of-function (LOF) phenotypes is rather limited in diploid cells, because if a mutation introduced into one allele, the second intact allele may serve as a backup. Therefore, recessive mutations are likely to be underrepresented among the screened phenotypes in diploid cells. For this reason, many global LOF screens were performed in haploid yeast, and only now when the derivation of haploid human ESCs, are available, it enables similar screens in human.
Genome-wide mutations can be introduced into haploid human ESCs by different methodologies. Libraries of mutant cells can then be subjected to different treatments followed by analysis of surviving clones, aiming to identify genes whose loss affords a growth advantage (positive selection) or disadvantage (negative selection). A similar approach is also applicable for haploid ESCs already carrying specific mutations, which can be isolated either from diseased eggs or generated by genome editing. In such cells, genome-wide mutagenesis can be performed to identify synthetic lethality interactions between the pre-existing mutation and a novel mutation within the library, based on mutual exclusivity. Alternatively, using diseased haploid human ESCs can reveal genes whose loss leads to reversion of the disease phenotype.

